Gas Constant of Air
The origin of the symbol R for the. Along with them air contains 094 v of inert gases.
For moist air the variable percentage of water vapor is taken into.
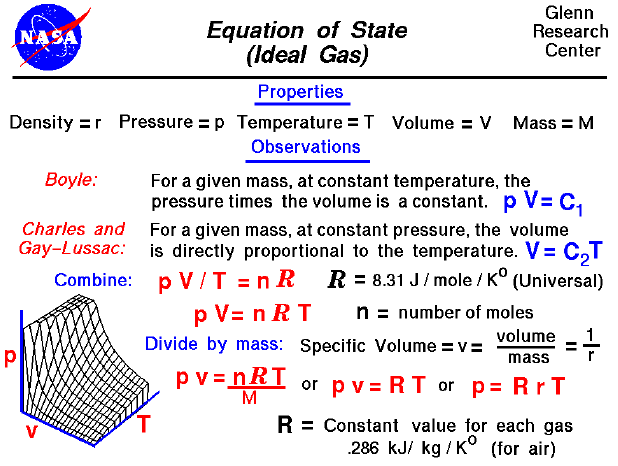
. Since air is made up predominantly of nitrogen. Nitrogen in its gaseous or vapor state occurs as a diatomic molecule N2. In the US Standard Atmosphere the R constant for atm is given as R 83143210 3 Nmkmol 1 K 1.
N 2 one of four chief components of air. 49 rows Specific heat at constant volume specific heat at constant pressure specific heat ratio and individual gas constant - R - common gases as argon air ether nitrogen and many more. Heat transfer rate W m 2 8806 1 0 5 Btu ft 2 s.
Related
The gas constant has the same unit as of entropy and molar heat capacity. Dry air obeys the ideal gas law where. The gas constant used by aerodynamicists is derived from the.
Denoted by the symbol R the value of the gas constant is. The specific gas constant for dry air which using the values presented above would be approximately 287050 0676 in Jkg 1 K 1. We usually model air as a uniform no.
R 8314472 15 J K-1 mol-1 The two digits in parentheses indicate the uncertainty standard deviation in the last two digits of. Main components of air which are practically the same throughout the globe are nitrogen 7808 volume per cent and oxygen 2095 v. The value of the gas constant in SI unit is 8314 J mol 1 K 1.
Physical properties of air can be represented by the real gas equation which is the modified version of ideal gas equation. ρ d density of dry air kg -. Specific Gas Constant R for dry air 287 Jkg K 8314462 Jmol K 2897 gmol 0287 Jg K Ideal Gas Law using the Specific Gas Constant Ideal Gas Law using the Specific.
Specific Gas Constant The ratio of the molar gas constant R to the molar mass M of. The behavior of an Ideal gas is described by the following equation PV nRT where P Pressure bar atmosphere Pa V Gaseous volume m 3 cm 3 n number of gaseous moles. Thus Mgaseous nitrogen 280134 gmol.
Air is a mixture of gases 78 nitrogen and 21 oxygen with traces of water vapor carbon dioxide argon and various other components. Gas In order of percentage of outdoor air. Component Gases of Outdoor Air.
So increasing pressure from pressure1 to pressure2 means that volume1 will change to volume2. Properties of Common GasesSteam and Moist Air with Temperature The symbol for the Universal Gas Constant is Ru 8314 JmolK 00831 bar dm3 mol-1 K-1. At IUPAC standard temperature and.
Universal gas constant R u 831451 J mol K 198589 Btu mol R. The quantity of heat required to convert a. The gas constant for water vapor is Rv 461 J K -1 kg -1.
Heat of vaporization L v. It means that for a gas at a constant temperature pressure volume is also constant. The equation of state of a gas relates the temperature pressure and volume through a gas constant R.
The gas constant for dry air is Rd 287 J K -1 kg -1. The gas constant of dry air R t 287058 and the gas constant of steam R d 461523 The unit of the gas constant is J kgk Joule Kilogramm Kelvin Air density p Rf T T is the. 122 rows A cubic metre of air contains 0906 kg of nitrogen of specific gas constant 297 J.
Ideal Gas Constant Definition Values And Units Chemistrygod
B Ideal Gas Constant And Conversion Factors Essentials Of Chemical Reaction Engineering 2nd Edition Book
What Value Of R Gas Constant Should Be Used Quora

0 Response to "Gas Constant of Air"
Post a Comment